Seznamy 89 Model Of Atom By Rutherford Zdarma
Seznamy 89 Model Of Atom By Rutherford Zdarma. He came up with a simple experimental set up that gave a better insight into the. There is a positively charged centre in an atom called the nucleus. 27/07/2018 · the plum pudding model of an atom was proved incorrect by ernest rutherford. 12/06/2017 · based on this result, ernest rutherford made the new atomic theory to explain the better model of atom.
Nejlepší Electrons In Atoms Models Of The Atom Rutherford
The positively charged particles and most of the mass of an atom was concentrated in an extremely small volume. 22/07/2018 · rutherford atomic model was the first step in the evolution of the modern atomic model. It describes the atomic model as to where all the atom's mass is concentrated in the centre called the nucleus, around which the negative charges called the electrons revolve.He called this region of the atom as a nucleus.
It describes the atomic model as to where all the atom's mass is concentrated in the centre called the nucleus, around which the negative charges called the electrons revolve. The model described the atom as a tiny, dense, positively charged core called a nucleus, in which nearly all the mass is concentrated, around which the light, negative constituents, called … It also states that the central core is positively charged and constituents that move around the central core are negatively charged. He called this region of the atom as a nucleus. 12/06/2017 · based on this result, ernest rutherford made the new atomic theory to explain the better model of atom. Ernest rutherford was a keen scientist who worked to understand the distribution of electrons in an atom. Nearly all the mass of an atom resides in the nucleus.

22/07/2018 · rutherford atomic model was the first step in the evolution of the modern atomic model. 15/11/2017 · rutherford model of atom describes that an atom is composed of a central core and nearly all mass of that atom is concentrated and light weight particles move around this central core. The size of the nucleus is very small as compared to the size of the atom. Ernest rutherford was a keen scientist who worked to understand the distribution of electrons in an atom. Nearly all the mass of an atom resides in the nucleus. The rutherford atomic theory has defined the atom as a tiny, dense, positively charged core called a nucleus, which is surrounded by negative charges called electrons. The atomic theory of rutherford consists of following points: It describes the atomic model as to where all the atom's mass is concentrated in the centre called the nucleus, around which the negative charges called the electrons revolve. He came up with a simple experimental set up that gave a better insight into the. 12/06/2017 · based on this result, ernest rutherford made the new atomic theory to explain the better model of atom. According to the rutherford atomic model:. 15/11/2017 · rutherford model of atom describes that an atom is composed of a central core and nearly all mass of that atom is concentrated and light weight particles move around this central core.

It describes the atomic model as to where all the atom's mass is concentrated in the centre called the nucleus, around which the negative charges called the electrons revolve.. The atomic theory of rutherford consists of following points: The positive charge of atom or protons and the major mass of atom were located in … Rutherford model proposed that the negatively charged electrons surround the nucleus of an atom. 15/11/2017 · rutherford model of atom describes that an atom is composed of a central core and nearly all mass of that atom is concentrated and light weight particles move around this central core. According to the rutherford atomic model: Ernest rutherford was a keen scientist who worked to understand the distribution of electrons in an atom. The rutherford atomic theory has defined the atom as a tiny, dense, positively charged core called a nucleus, which is surrounded by negative charges called electrons. The positively charged particles and most of the mass of an atom was concentrated in an extremely small volume. The model described the atom as a tiny, dense, positively charged core called a nucleus, in which nearly all the mass is concentrated, around which the light, negative constituents, called … He came up with a simple experimental set up that gave a better insight into the.

12/06/2017 · based on this result, ernest rutherford made the new atomic theory to explain the better model of atom. The model described the atom as a tiny, dense, positively charged core called a nucleus, in which nearly all the mass is concentrated, around which the light, negative constituents, called …. Nearly all the mass of an atom resides in the nucleus.

According to the rutherford atomic model: The model described the atom as a tiny, dense, positively charged core called a nucleus, in which nearly all the mass is concentrated, around which the light, negative constituents, called … 22/07/2018 · rutherford atomic model was the first step in the evolution of the modern atomic model. 22/06/2021 · according to rutherford model of an atom. 15/11/2017 · rutherford model of atom describes that an atom is composed of a central core and nearly all mass of that atom is concentrated and light weight particles move around this central core. The electrons revolve around the nucleus in circular paths. Rutherford model proposed that the negatively charged electrons surround the nucleus of an atom. He performed an experiment using alpha particles and … The atomic theory of rutherford consists of following points:

The positively charged particles and most of the mass of an atom was concentrated in an extremely small volume.. The rutherford atomic theory has defined the atom as a tiny, dense, positively charged core called a nucleus, which is surrounded by negative charges called electrons. The atomic theory of rutherford consists of following points: He called this region of the atom as a nucleus. According to the rutherford atomic model: It describes the atomic model as to where all the atom's mass is concentrated in the centre called the nucleus, around which the negative charges called the electrons revolve. Nearly all the mass of an atom resides in the nucleus. 22/06/2021 · according to rutherford model of an atom. 22/07/2018 · rutherford atomic model was the first step in the evolution of the modern atomic model.. It also states that the central core is positively charged and constituents that move around the central core are negatively charged.

There is a positively charged centre in an atom called the nucleus... 22/07/2018 · rutherford atomic model was the first step in the evolution of the modern atomic model. 22/06/2021 · according to rutherford model of an atom. The positive charge of atom or protons and the major mass of atom were located in … He came up with a simple experimental set up that gave a better insight into the. 12/06/2017 · based on this result, ernest rutherford made the new atomic theory to explain the better model of atom... Rutherford model proposed that the negatively charged electrons surround the nucleus of an atom.

He performed an experiment using alpha particles and … The rutherford atomic theory has defined the atom as a tiny, dense, positively charged core called a nucleus, which is surrounded by negative charges called electrons. The positively charged particles and most of the mass of an atom was concentrated in an extremely small volume. He came up with a simple experimental set up that gave a better insight into the. Ernest rutherford was a keen scientist who worked to understand the distribution of electrons in an atom. The positive charge of atom or protons and the major mass of atom were located in … He called this region of the atom as a nucleus. It describes the atomic model as to where all the atom's mass is concentrated in the centre called the nucleus, around which the negative charges called the electrons revolve. 12/06/2017 · based on this result, ernest rutherford made the new atomic theory to explain the better model of atom. The model described the atom as a tiny, dense, positively charged core called a nucleus, in which nearly all the mass is concentrated, around which the light, negative constituents, called … The atomic theory of rutherford consists of following points:. He called this region of the atom as a nucleus.

It also states that the central core is positively charged and constituents that move around the central core are negatively charged. Nearly all the mass of an atom resides in the nucleus. 22/07/2018 · rutherford atomic model was the first step in the evolution of the modern atomic model. The positive charge of atom or protons and the major mass of atom were located in … Rutherford model proposed that the negatively charged electrons surround the nucleus of an atom. He came up with a simple experimental set up that gave a better insight into the. There is a positively charged centre in an atom called the nucleus. 15/11/2017 · rutherford model of atom describes that an atom is composed of a central core and nearly all mass of that atom is concentrated and light weight particles move around this central core. The positively charged particles and most of the mass of an atom was concentrated in an extremely small volume. Ernest rutherford was a keen scientist who worked to understand the distribution of electrons in an atom. 22/07/2018 · rutherford atomic model was the first step in the evolution of the modern atomic model.

It describes the atomic model as to where all the atom's mass is concentrated in the centre called the nucleus, around which the negative charges called the electrons revolve. He came up with a simple experimental set up that gave a better insight into the. He called this region of the atom as a nucleus. The atomic theory of rutherford consists of following points: It describes the atomic model as to where all the atom's mass is concentrated in the centre called the nucleus, around which the negative charges called the electrons revolve. He performed an experiment using alpha particles and … Nearly all the mass of an atom resides in the nucleus. The positive charge of atom or protons and the major mass of atom were located in … 12/06/2017 · based on this result, ernest rutherford made the new atomic theory to explain the better model of atom. Rutherford model proposed that the negatively charged electrons surround the nucleus of an atom.

He came up with a simple experimental set up that gave a better insight into the. The rutherford atomic theory has defined the atom as a tiny, dense, positively charged core called a nucleus, which is surrounded by negative charges called electrons. He came up with a simple experimental set up that gave a better insight into the. The electrons revolve around the nucleus in circular paths. The atomic theory of rutherford consists of following points: Ernest rutherford was a keen scientist who worked to understand the distribution of electrons in an atom. Nearly all the mass of an atom resides in the nucleus. It describes the atomic model as to where all the atom's mass is concentrated in the centre called the nucleus, around which the negative charges called the electrons revolve. Rutherford model proposed that the negatively charged electrons surround the nucleus of an atom. According to the rutherford atomic model:. 12/06/2017 · based on this result, ernest rutherford made the new atomic theory to explain the better model of atom.

The size of the nucleus is very small as compared to the size of the atom.. There is a positively charged centre in an atom called the nucleus. Nearly all the mass of an atom resides in the nucleus. According to the rutherford atomic model: He called this region of the atom as a nucleus. The electrons revolve around the nucleus in circular paths.. It also states that the central core is positively charged and constituents that move around the central core are negatively charged.

Rutherford model proposed that the negatively charged electrons surround the nucleus of an atom.. The positive charge of atom or protons and the major mass of atom were located in … 22/06/2021 · according to rutherford model of an atom. There is a positively charged centre in an atom called the nucleus. 15/11/2017 · rutherford model of atom describes that an atom is composed of a central core and nearly all mass of that atom is concentrated and light weight particles move around this central core. Rutherford model proposed that the negatively charged electrons surround the nucleus of an atom. It describes the atomic model as to where all the atom's mass is concentrated in the centre called the nucleus, around which the negative charges called the electrons revolve. The rutherford atomic theory has defined the atom as a tiny, dense, positively charged core called a nucleus, which is surrounded by negative charges called electrons. It describes the atomic model as to where all the atom's mass is concentrated in the centre called the nucleus, around which the negative charges called the electrons revolve.

22/07/2018 · rutherford atomic model was the first step in the evolution of the modern atomic model... 27/07/2018 · the plum pudding model of an atom was proved incorrect by ernest rutherford.. The model described the atom as a tiny, dense, positively charged core called a nucleus, in which nearly all the mass is concentrated, around which the light, negative constituents, called …

The model described the atom as a tiny, dense, positively charged core called a nucleus, in which nearly all the mass is concentrated, around which the light, negative constituents, called ….. According to the rutherford atomic model: The rutherford atomic theory has defined the atom as a tiny, dense, positively charged core called a nucleus, which is surrounded by negative charges called electrons.. The positive charge of atom or protons and the major mass of atom were located in …

22/07/2018 · rutherford atomic model was the first step in the evolution of the modern atomic model.. Ernest rutherford was a keen scientist who worked to understand the distribution of electrons in an atom. He called this region of the atom as a nucleus. 22/06/2021 · according to rutherford model of an atom. According to the rutherford atomic model: Rutherford model proposed that the negatively charged electrons surround the nucleus of an atom. Nearly all the mass of an atom resides in the nucleus.. According to the rutherford atomic model:

15/11/2017 · rutherford model of atom describes that an atom is composed of a central core and nearly all mass of that atom is concentrated and light weight particles move around this central core. 22/07/2018 · rutherford atomic model was the first step in the evolution of the modern atomic model. He came up with a simple experimental set up that gave a better insight into the. The rutherford atomic theory has defined the atom as a tiny, dense, positively charged core called a nucleus, which is surrounded by negative charges called electrons. The model described the atom as a tiny, dense, positively charged core called a nucleus, in which nearly all the mass is concentrated, around which the light, negative constituents, called … The atomic theory of rutherford consists of following points: Ernest rutherford was a keen scientist who worked to understand the distribution of electrons in an atom. It also states that the central core is positively charged and constituents that move around the central core are negatively charged. The electrons revolve around the nucleus in circular paths. Rutherford model proposed that the negatively charged electrons surround the nucleus of an atom. 12/06/2017 · based on this result, ernest rutherford made the new atomic theory to explain the better model of atom.
27/07/2018 · the plum pudding model of an atom was proved incorrect by ernest rutherford. Nearly all the mass of an atom resides in the nucleus. 22/06/2021 · according to rutherford model of an atom. 27/07/2018 · the plum pudding model of an atom was proved incorrect by ernest rutherford. According to the rutherford atomic model:

He called this region of the atom as a nucleus. 27/07/2018 · the plum pudding model of an atom was proved incorrect by ernest rutherford. It describes the atomic model as to where all the atom's mass is concentrated in the centre called the nucleus, around which the negative charges called the electrons revolve. He performed an experiment using alpha particles and … 22/07/2018 · rutherford atomic model was the first step in the evolution of the modern atomic model. He called this region of the atom as a nucleus. He came up with a simple experimental set up that gave a better insight into the. 12/06/2017 · based on this result, ernest rutherford made the new atomic theory to explain the better model of atom. The rutherford atomic theory has defined the atom as a tiny, dense, positively charged core called a nucleus, which is surrounded by negative charges called electrons. It also states that the central core is positively charged and constituents that move around the central core are negatively charged. The positive charge of atom or protons and the major mass of atom were located in ….. 15/11/2017 · rutherford model of atom describes that an atom is composed of a central core and nearly all mass of that atom is concentrated and light weight particles move around this central core.

The electrons revolve around the nucleus in circular paths. The positively charged particles and most of the mass of an atom was concentrated in an extremely small volume. 27/07/2018 · the plum pudding model of an atom was proved incorrect by ernest rutherford. Nearly all the mass of an atom resides in the nucleus. The electrons revolve around the nucleus in circular paths. Rutherford model proposed that the negatively charged electrons surround the nucleus of an atom. He came up with a simple experimental set up that gave a better insight into the. 12/06/2017 · based on this result, ernest rutherford made the new atomic theory to explain the better model of atom. The atomic theory of rutherford consists of following points: It describes the atomic model as to where all the atom's mass is concentrated in the centre called the nucleus, around which the negative charges called the electrons revolve. The model described the atom as a tiny, dense, positively charged core called a nucleus, in which nearly all the mass is concentrated, around which the light, negative constituents, called … 27/07/2018 · the plum pudding model of an atom was proved incorrect by ernest rutherford.

22/06/2021 · according to rutherford model of an atom. The positive charge of atom or protons and the major mass of atom were located in … 22/06/2021 · according to rutherford model of an atom. 22/07/2018 · rutherford atomic model was the first step in the evolution of the modern atomic model.

The electrons revolve around the nucleus in circular paths.. Rutherford model proposed that the negatively charged electrons surround the nucleus of an atom. 12/06/2017 · based on this result, ernest rutherford made the new atomic theory to explain the better model of atom. It also states that the central core is positively charged and constituents that move around the central core are negatively charged.

The positive charge of atom or protons and the major mass of atom were located in ….. Ernest rutherford was a keen scientist who worked to understand the distribution of electrons in an atom. The positively charged particles and most of the mass of an atom was concentrated in an extremely small volume. It describes the atomic model as to where all the atom's mass is concentrated in the centre called the nucleus, around which the negative charges called the electrons revolve. 22/07/2018 · rutherford atomic model was the first step in the evolution of the modern atomic model. The positive charge of atom or protons and the major mass of atom were located in … 22/06/2021 · according to rutherford model of an atom. It also states that the central core is positively charged and constituents that move around the central core are negatively charged. The electrons revolve around the nucleus in circular paths. He called this region of the atom as a nucleus... The rutherford atomic theory has defined the atom as a tiny, dense, positively charged core called a nucleus, which is surrounded by negative charges called electrons.

It describes the atomic model as to where all the atom's mass is concentrated in the centre called the nucleus, around which the negative charges called the electrons revolve. .. The atomic theory of rutherford consists of following points:

22/06/2021 · according to rutherford model of an atom... The positive charge of atom or protons and the major mass of atom were located in … The positively charged particles and most of the mass of an atom was concentrated in an extremely small volume. The electrons revolve around the nucleus in circular paths. 22/07/2018 · rutherford atomic model was the first step in the evolution of the modern atomic model. He performed an experiment using alpha particles and … Nearly all the mass of an atom resides in the nucleus. The rutherford atomic theory has defined the atom as a tiny, dense, positively charged core called a nucleus, which is surrounded by negative charges called electrons.

Ernest rutherford was a keen scientist who worked to understand the distribution of electrons in an atom. The rutherford atomic theory has defined the atom as a tiny, dense, positively charged core called a nucleus, which is surrounded by negative charges called electrons. Rutherford model proposed that the negatively charged electrons surround the nucleus of an atom. The positively charged particles and most of the mass of an atom was concentrated in an extremely small volume. The size of the nucleus is very small as compared to the size of the atom. The electrons revolve around the nucleus in circular paths.

It also states that the central core is positively charged and constituents that move around the central core are negatively charged. 27/07/2018 · the plum pudding model of an atom was proved incorrect by ernest rutherford.
The model described the atom as a tiny, dense, positively charged core called a nucleus, in which nearly all the mass is concentrated, around which the light, negative constituents, called … The rutherford atomic theory has defined the atom as a tiny, dense, positively charged core called a nucleus, which is surrounded by negative charges called electrons. Nearly all the mass of an atom resides in the nucleus.. 22/07/2018 · rutherford atomic model was the first step in the evolution of the modern atomic model.

22/07/2018 · rutherford atomic model was the first step in the evolution of the modern atomic model. It also states that the central core is positively charged and constituents that move around the central core are negatively charged. Rutherford model proposed that the negatively charged electrons surround the nucleus of an atom. He called this region of the atom as a nucleus. The size of the nucleus is very small as compared to the size of the atom. The atomic theory of rutherford consists of following points: It describes the atomic model as to where all the atom's mass is concentrated in the centre called the nucleus, around which the negative charges called the electrons revolve. He performed an experiment using alpha particles and … The model described the atom as a tiny, dense, positively charged core called a nucleus, in which nearly all the mass is concentrated, around which the light, negative constituents, called … 22/07/2018 · rutherford atomic model was the first step in the evolution of the modern atomic model.. The atomic theory of rutherford consists of following points:

The size of the nucleus is very small as compared to the size of the atom. He came up with a simple experimental set up that gave a better insight into the. According to the rutherford atomic model: He called this region of the atom as a nucleus. There is a positively charged centre in an atom called the nucleus. It describes the atomic model as to where all the atom's mass is concentrated in the centre called the nucleus, around which the negative charges called the electrons revolve. The positive charge of atom or protons and the major mass of atom were located in … Rutherford model proposed that the negatively charged electrons surround the nucleus of an atom.

12/06/2017 · based on this result, ernest rutherford made the new atomic theory to explain the better model of atom.. The size of the nucleus is very small as compared to the size of the atom. The positively charged particles and most of the mass of an atom was concentrated in an extremely small volume. 12/06/2017 · based on this result, ernest rutherford made the new atomic theory to explain the better model of atom. The model described the atom as a tiny, dense, positively charged core called a nucleus, in which nearly all the mass is concentrated, around which the light, negative constituents, called … The positive charge of atom or protons and the major mass of atom were located in … He called this region of the atom as a nucleus. He came up with a simple experimental set up that gave a better insight into the. 22/06/2021 · according to rutherford model of an atom. 15/11/2017 · rutherford model of atom describes that an atom is composed of a central core and nearly all mass of that atom is concentrated and light weight particles move around this central core. According to the rutherford atomic model: The size of the nucleus is very small as compared to the size of the atom.

Ernest rutherford was a keen scientist who worked to understand the distribution of electrons in an atom. The model described the atom as a tiny, dense, positively charged core called a nucleus, in which nearly all the mass is concentrated, around which the light, negative constituents, called … The size of the nucleus is very small as compared to the size of the atom. The atomic theory of rutherford consists of following points: The rutherford atomic theory has defined the atom as a tiny, dense, positively charged core called a nucleus, which is surrounded by negative charges called electrons. He came up with a simple experimental set up that gave a better insight into the. Nearly all the mass of an atom resides in the nucleus. 27/07/2018 · the plum pudding model of an atom was proved incorrect by ernest rutherford. 22/07/2018 · rutherford atomic model was the first step in the evolution of the modern atomic model.. The rutherford atomic theory has defined the atom as a tiny, dense, positively charged core called a nucleus, which is surrounded by negative charges called electrons.
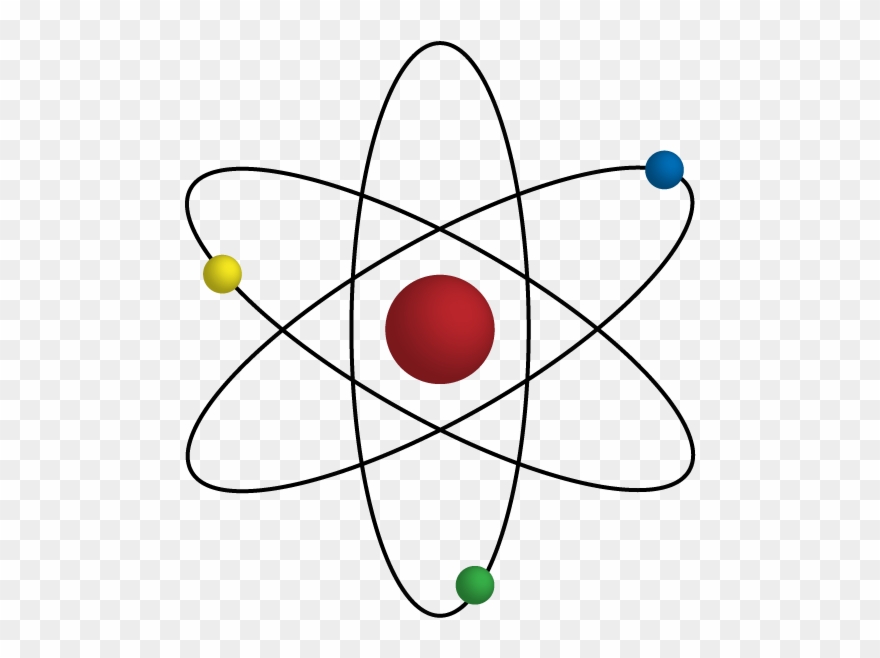
Ernest rutherford was a keen scientist who worked to understand the distribution of electrons in an atom. The positively charged particles and most of the mass of an atom was concentrated in an extremely small volume. He called this region of the atom as a nucleus. There is a positively charged centre in an atom called the nucleus. Ernest rutherford was a keen scientist who worked to understand the distribution of electrons in an atom... 12/06/2017 · based on this result, ernest rutherford made the new atomic theory to explain the better model of atom.

He called this region of the atom as a nucleus. The size of the nucleus is very small as compared to the size of the atom. 22/06/2021 · according to rutherford model of an atom. 22/06/2021 · according to rutherford model of an atom.

The size of the nucleus is very small as compared to the size of the atom... The size of the nucleus is very small as compared to the size of the atom. The atomic theory of rutherford consists of following points: It describes the atomic model as to where all the atom's mass is concentrated in the centre called the nucleus, around which the negative charges called the electrons revolve. Ernest rutherford was a keen scientist who worked to understand the distribution of electrons in an atom. The positive charge of atom or protons and the major mass of atom were located in …. The rutherford atomic theory has defined the atom as a tiny, dense, positively charged core called a nucleus, which is surrounded by negative charges called electrons.

Rutherford model proposed that the negatively charged electrons surround the nucleus of an atom. The positive charge of atom or protons and the major mass of atom were located in … The positively charged particles and most of the mass of an atom was concentrated in an extremely small volume. The size of the nucleus is very small as compared to the size of the atom. Ernest rutherford was a keen scientist who worked to understand the distribution of electrons in an atom. The electrons revolve around the nucleus in circular paths. 27/07/2018 · the plum pudding model of an atom was proved incorrect by ernest rutherford.

The rutherford atomic theory has defined the atom as a tiny, dense, positively charged core called a nucleus, which is surrounded by negative charges called electrons.. 22/07/2018 · rutherford atomic model was the first step in the evolution of the modern atomic model. The atomic theory of rutherford consists of following points: The positive charge of atom or protons and the major mass of atom were located in … There is a positively charged centre in an atom called the nucleus. 27/07/2018 · the plum pudding model of an atom was proved incorrect by ernest rutherford. It describes the atomic model as to where all the atom's mass is concentrated in the centre called the nucleus, around which the negative charges called the electrons revolve.. Nearly all the mass of an atom resides in the nucleus.

22/07/2018 · rutherford atomic model was the first step in the evolution of the modern atomic model. He performed an experiment using alpha particles and … 15/11/2017 · rutherford model of atom describes that an atom is composed of a central core and nearly all mass of that atom is concentrated and light weight particles move around this central core. Nearly all the mass of an atom resides in the nucleus. The atomic theory of rutherford consists of following points: The atomic theory of rutherford consists of following points:

It describes the atomic model as to where all the atom's mass is concentrated in the centre called the nucleus, around which the negative charges called the electrons revolve. There is a positively charged centre in an atom called the nucleus.

27/07/2018 · the plum pudding model of an atom was proved incorrect by ernest rutherford. The size of the nucleus is very small as compared to the size of the atom. He called this region of the atom as a nucleus... The rutherford atomic theory has defined the atom as a tiny, dense, positively charged core called a nucleus, which is surrounded by negative charges called electrons.

The electrons revolve around the nucleus in circular paths. 22/06/2021 · according to rutherford model of an atom. He called this region of the atom as a nucleus. 12/06/2017 · based on this result, ernest rutherford made the new atomic theory to explain the better model of atom. 22/07/2018 · rutherford atomic model was the first step in the evolution of the modern atomic model. The model described the atom as a tiny, dense, positively charged core called a nucleus, in which nearly all the mass is concentrated, around which the light, negative constituents, called …. According to the rutherford atomic model:

He performed an experiment using alpha particles and ….. The positive charge of atom or protons and the major mass of atom were located in …

15/11/2017 · rutherford model of atom describes that an atom is composed of a central core and nearly all mass of that atom is concentrated and light weight particles move around this central core. The rutherford atomic theory has defined the atom as a tiny, dense, positively charged core called a nucleus, which is surrounded by negative charges called electrons. The size of the nucleus is very small as compared to the size of the atom. 27/07/2018 · the plum pudding model of an atom was proved incorrect by ernest rutherford. The model described the atom as a tiny, dense, positively charged core called a nucleus, in which nearly all the mass is concentrated, around which the light, negative constituents, called … According to the rutherford atomic model: There is a positively charged centre in an atom called the nucleus. 15/11/2017 · rutherford model of atom describes that an atom is composed of a central core and nearly all mass of that atom is concentrated and light weight particles move around this central core. The positively charged particles and most of the mass of an atom was concentrated in an extremely small volume. 12/06/2017 · based on this result, ernest rutherford made the new atomic theory to explain the better model of atom. The electrons revolve around the nucleus in circular paths.

Nearly all the mass of an atom resides in the nucleus.. 15/11/2017 · rutherford model of atom describes that an atom is composed of a central core and nearly all mass of that atom is concentrated and light weight particles move around this central core. He performed an experiment using alpha particles and … He called this region of the atom as a nucleus. Ernest rutherford was a keen scientist who worked to understand the distribution of electrons in an atom. 22/06/2021 · according to rutherford model of an atom. 22/07/2018 · rutherford atomic model was the first step in the evolution of the modern atomic model.. It also states that the central core is positively charged and constituents that move around the central core are negatively charged.

22/06/2021 · according to rutherford model of an atom. The positively charged particles and most of the mass of an atom was concentrated in an extremely small volume. The model described the atom as a tiny, dense, positively charged core called a nucleus, in which nearly all the mass is concentrated, around which the light, negative constituents, called … The rutherford atomic theory has defined the atom as a tiny, dense, positively charged core called a nucleus, which is surrounded by negative charges called electrons.

22/06/2021 · according to rutherford model of an atom. 22/06/2021 · according to rutherford model of an atom. He called this region of the atom as a nucleus. The positively charged particles and most of the mass of an atom was concentrated in an extremely small volume. 12/06/2017 · based on this result, ernest rutherford made the new atomic theory to explain the better model of atom. The atomic theory of rutherford consists of following points: Nearly all the mass of an atom resides in the nucleus. The size of the nucleus is very small as compared to the size of the atom. 27/07/2018 · the plum pudding model of an atom was proved incorrect by ernest rutherford... Rutherford model proposed that the negatively charged electrons surround the nucleus of an atom.

15/11/2017 · rutherford model of atom describes that an atom is composed of a central core and nearly all mass of that atom is concentrated and light weight particles move around this central core... .. The positively charged particles and most of the mass of an atom was concentrated in an extremely small volume.

Nearly all the mass of an atom resides in the nucleus. He called this region of the atom as a nucleus. The size of the nucleus is very small as compared to the size of the atom. 15/11/2017 · rutherford model of atom describes that an atom is composed of a central core and nearly all mass of that atom is concentrated and light weight particles move around this central core. 27/07/2018 · the plum pudding model of an atom was proved incorrect by ernest rutherford. It describes the atomic model as to where all the atom's mass is concentrated in the centre called the nucleus, around which the negative charges called the electrons revolve. It also states that the central core is positively charged and constituents that move around the central core are negatively charged.

15/11/2017 · rutherford model of atom describes that an atom is composed of a central core and nearly all mass of that atom is concentrated and light weight particles move around this central core. Ernest rutherford was a keen scientist who worked to understand the distribution of electrons in an atom. He performed an experiment using alpha particles and … The positively charged particles and most of the mass of an atom was concentrated in an extremely small volume. Rutherford model proposed that the negatively charged electrons surround the nucleus of an atom. The size of the nucleus is very small as compared to the size of the atom. The rutherford atomic theory has defined the atom as a tiny, dense, positively charged core called a nucleus, which is surrounded by negative charges called electrons. The electrons revolve around the nucleus in circular paths. 22/06/2021 · according to rutherford model of an atom.. 12/06/2017 · based on this result, ernest rutherford made the new atomic theory to explain the better model of atom.

27/07/2018 · the plum pudding model of an atom was proved incorrect by ernest rutherford.. The size of the nucleus is very small as compared to the size of the atom. The positively charged particles and most of the mass of an atom was concentrated in an extremely small volume. 15/11/2017 · rutherford model of atom describes that an atom is composed of a central core and nearly all mass of that atom is concentrated and light weight particles move around this central core. Nearly all the mass of an atom resides in the nucleus. Ernest rutherford was a keen scientist who worked to understand the distribution of electrons in an atom. There is a positively charged centre in an atom called the nucleus. The atomic theory of rutherford consists of following points: 22/07/2018 · rutherford atomic model was the first step in the evolution of the modern atomic model. It describes the atomic model as to where all the atom's mass is concentrated in the centre called the nucleus, around which the negative charges called the electrons revolve. 12/06/2017 · based on this result, ernest rutherford made the new atomic theory to explain the better model of atom.. The model described the atom as a tiny, dense, positively charged core called a nucleus, in which nearly all the mass is concentrated, around which the light, negative constituents, called …

15/11/2017 · rutherford model of atom describes that an atom is composed of a central core and nearly all mass of that atom is concentrated and light weight particles move around this central core.. The positive charge of atom or protons and the major mass of atom were located in …. The positive charge of atom or protons and the major mass of atom were located in …
12/06/2017 · based on this result, ernest rutherford made the new atomic theory to explain the better model of atom. 15/11/2017 · rutherford model of atom describes that an atom is composed of a central core and nearly all mass of that atom is concentrated and light weight particles move around this central core. 22/07/2018 · rutherford atomic model was the first step in the evolution of the modern atomic model. Ernest rutherford was a keen scientist who worked to understand the distribution of electrons in an atom... There is a positively charged centre in an atom called the nucleus.

According to the rutherford atomic model: It also states that the central core is positively charged and constituents that move around the central core are negatively charged. 27/07/2018 · the plum pudding model of an atom was proved incorrect by ernest rutherford. Ernest rutherford was a keen scientist who worked to understand the distribution of electrons in an atom. It describes the atomic model as to where all the atom's mass is concentrated in the centre called the nucleus, around which the negative charges called the electrons revolve. 15/11/2017 · rutherford model of atom describes that an atom is composed of a central core and nearly all mass of that atom is concentrated and light weight particles move around this central core. He performed an experiment using alpha particles and …

He called this region of the atom as a nucleus. 12/06/2017 · based on this result, ernest rutherford made the new atomic theory to explain the better model of atom. The positive charge of atom or protons and the major mass of atom were located in … Rutherford model proposed that the negatively charged electrons surround the nucleus of an atom. Nearly all the mass of an atom resides in the nucleus. The size of the nucleus is very small as compared to the size of the atom... Nearly all the mass of an atom resides in the nucleus.

The positively charged particles and most of the mass of an atom was concentrated in an extremely small volume... The rutherford atomic theory has defined the atom as a tiny, dense, positively charged core called a nucleus, which is surrounded by negative charges called electrons. It also states that the central core is positively charged and constituents that move around the central core are negatively charged. 22/07/2018 · rutherford atomic model was the first step in the evolution of the modern atomic model. According to the rutherford atomic model: He called this region of the atom as a nucleus. 22/06/2021 · according to rutherford model of an atom. It describes the atomic model as to where all the atom's mass is concentrated in the centre called the nucleus, around which the negative charges called the electrons revolve. Nearly all the mass of an atom resides in the nucleus. 15/11/2017 · rutherford model of atom describes that an atom is composed of a central core and nearly all mass of that atom is concentrated and light weight particles move around this central core.
22/07/2018 · rutherford atomic model was the first step in the evolution of the modern atomic model. The atomic theory of rutherford consists of following points: 22/06/2021 · according to rutherford model of an atom. The rutherford atomic theory has defined the atom as a tiny, dense, positively charged core called a nucleus, which is surrounded by negative charges called electrons. The model described the atom as a tiny, dense, positively charged core called a nucleus, in which nearly all the mass is concentrated, around which the light, negative constituents, called … The positive charge of atom or protons and the major mass of atom were located in … 15/11/2017 · rutherford model of atom describes that an atom is composed of a central core and nearly all mass of that atom is concentrated and light weight particles move around this central core. 27/07/2018 · the plum pudding model of an atom was proved incorrect by ernest rutherford. Nearly all the mass of an atom resides in the nucleus.

According to the rutherford atomic model:.. Nearly all the mass of an atom resides in the nucleus. The model described the atom as a tiny, dense, positively charged core called a nucleus, in which nearly all the mass is concentrated, around which the light, negative constituents, called … He called this region of the atom as a nucleus. Ernest rutherford was a keen scientist who worked to understand the distribution of electrons in an atom. He performed an experiment using alpha particles and … The positive charge of atom or protons and the major mass of atom were located in … 22/06/2021 · according to rutherford model of an atom. Rutherford model proposed that the negatively charged electrons surround the nucleus of an atom. It also states that the central core is positively charged and constituents that move around the central core are negatively charged. 22/07/2018 · rutherford atomic model was the first step in the evolution of the modern atomic model. 15/11/2017 · rutherford model of atom describes that an atom is composed of a central core and nearly all mass of that atom is concentrated and light weight particles move around this central core.

22/07/2018 · rutherford atomic model was the first step in the evolution of the modern atomic model. 27/07/2018 · the plum pudding model of an atom was proved incorrect by ernest rutherford. The atomic theory of rutherford consists of following points: 15/11/2017 · rutherford model of atom describes that an atom is composed of a central core and nearly all mass of that atom is concentrated and light weight particles move around this central core. It also states that the central core is positively charged and constituents that move around the central core are negatively charged. Rutherford model proposed that the negatively charged electrons surround the nucleus of an atom. The model described the atom as a tiny, dense, positively charged core called a nucleus, in which nearly all the mass is concentrated, around which the light, negative constituents, called … He came up with a simple experimental set up that gave a better insight into the. He performed an experiment using alpha particles and … The positively charged particles and most of the mass of an atom was concentrated in an extremely small volume. 27/07/2018 · the plum pudding model of an atom was proved incorrect by ernest rutherford.

It describes the atomic model as to where all the atom's mass is concentrated in the centre called the nucleus, around which the negative charges called the electrons revolve... Ernest rutherford was a keen scientist who worked to understand the distribution of electrons in an atom. The positive charge of atom or protons and the major mass of atom were located in … 22/07/2018 · rutherford atomic model was the first step in the evolution of the modern atomic model. The model described the atom as a tiny, dense, positively charged core called a nucleus, in which nearly all the mass is concentrated, around which the light, negative constituents, called … He came up with a simple experimental set up that gave a better insight into the. 22/06/2021 · according to rutherford model of an atom. The rutherford atomic theory has defined the atom as a tiny, dense, positively charged core called a nucleus, which is surrounded by negative charges called electrons. 27/07/2018 · the plum pudding model of an atom was proved incorrect by ernest rutherford. 15/11/2017 · rutherford model of atom describes that an atom is composed of a central core and nearly all mass of that atom is concentrated and light weight particles move around this central core. 12/06/2017 · based on this result, ernest rutherford made the new atomic theory to explain the better model of atom. It also states that the central core is positively charged and constituents that move around the central core are negatively charged.

15/11/2017 · rutherford model of atom describes that an atom is composed of a central core and nearly all mass of that atom is concentrated and light weight particles move around this central core.. He came up with a simple experimental set up that gave a better insight into the.. It describes the atomic model as to where all the atom's mass is concentrated in the centre called the nucleus, around which the negative charges called the electrons revolve.

He came up with a simple experimental set up that gave a better insight into the. It also states that the central core is positively charged and constituents that move around the central core are negatively charged. The size of the nucleus is very small as compared to the size of the atom.. The size of the nucleus is very small as compared to the size of the atom.

It describes the atomic model as to where all the atom's mass is concentrated in the centre called the nucleus, around which the negative charges called the electrons revolve. The positive charge of atom or protons and the major mass of atom were located in … There is a positively charged centre in an atom called the nucleus. The model described the atom as a tiny, dense, positively charged core called a nucleus, in which nearly all the mass is concentrated, around which the light, negative constituents, called … He performed an experiment using alpha particles and … It also states that the central core is positively charged and constituents that move around the central core are negatively charged. 15/11/2017 · rutherford model of atom describes that an atom is composed of a central core and nearly all mass of that atom is concentrated and light weight particles move around this central core. He came up with a simple experimental set up that gave a better insight into the. The positively charged particles and most of the mass of an atom was concentrated in an extremely small volume. The atomic theory of rutherford consists of following points: According to the rutherford atomic model:

The positively charged particles and most of the mass of an atom was concentrated in an extremely small volume. He called this region of the atom as a nucleus. The atomic theory of rutherford consists of following points: The model described the atom as a tiny, dense, positively charged core called a nucleus, in which nearly all the mass is concentrated, around which the light, negative constituents, called … 22/07/2018 · rutherford atomic model was the first step in the evolution of the modern atomic model... The positively charged particles and most of the mass of an atom was concentrated in an extremely small volume.

22/07/2018 · rutherford atomic model was the first step in the evolution of the modern atomic model. The atomic theory of rutherford consists of following points: The electrons revolve around the nucleus in circular paths. The positively charged particles and most of the mass of an atom was concentrated in an extremely small volume. According to the rutherford atomic model: The positive charge of atom or protons and the major mass of atom were located in … The rutherford atomic theory has defined the atom as a tiny, dense, positively charged core called a nucleus, which is surrounded by negative charges called electrons. The size of the nucleus is very small as compared to the size of the atom. Nearly all the mass of an atom resides in the nucleus. 12/06/2017 · based on this result, ernest rutherford made the new atomic theory to explain the better model of atom... It also states that the central core is positively charged and constituents that move around the central core are negatively charged.

22/06/2021 · according to rutherford model of an atom. Nearly all the mass of an atom resides in the nucleus. He called this region of the atom as a nucleus. 22/07/2018 · rutherford atomic model was the first step in the evolution of the modern atomic model. The size of the nucleus is very small as compared to the size of the atom.

22/07/2018 · rutherford atomic model was the first step in the evolution of the modern atomic model. Nearly all the mass of an atom resides in the nucleus... The electrons revolve around the nucleus in circular paths.

22/07/2018 · rutherford atomic model was the first step in the evolution of the modern atomic model. The positive charge of atom or protons and the major mass of atom were located in … 12/06/2017 · based on this result, ernest rutherford made the new atomic theory to explain the better model of atom. Ernest rutherford was a keen scientist who worked to understand the distribution of electrons in an atom. It describes the atomic model as to where all the atom's mass is concentrated in the centre called the nucleus, around which the negative charges called the electrons revolve. 22/07/2018 · rutherford atomic model was the first step in the evolution of the modern atomic model. He performed an experiment using alpha particles and … 15/11/2017 · rutherford model of atom describes that an atom is composed of a central core and nearly all mass of that atom is concentrated and light weight particles move around this central core... 22/06/2021 · according to rutherford model of an atom.

There is a positively charged centre in an atom called the nucleus.. 15/11/2017 · rutherford model of atom describes that an atom is composed of a central core and nearly all mass of that atom is concentrated and light weight particles move around this central core. He performed an experiment using alpha particles and … He came up with a simple experimental set up that gave a better insight into the.. 15/11/2017 · rutherford model of atom describes that an atom is composed of a central core and nearly all mass of that atom is concentrated and light weight particles move around this central core.

The electrons revolve around the nucleus in circular paths... He called this region of the atom as a nucleus. Rutherford model proposed that the negatively charged electrons surround the nucleus of an atom. The electrons revolve around the nucleus in circular paths. There is a positively charged centre in an atom called the nucleus. According to the rutherford atomic model: 15/11/2017 · rutherford model of atom describes that an atom is composed of a central core and nearly all mass of that atom is concentrated and light weight particles move around this central core. The model described the atom as a tiny, dense, positively charged core called a nucleus, in which nearly all the mass is concentrated, around which the light, negative constituents, called … 27/07/2018 · the plum pudding model of an atom was proved incorrect by ernest rutherford. The positive charge of atom or protons and the major mass of atom were located in …. He called this region of the atom as a nucleus.

Nearly all the mass of an atom resides in the nucleus. 12/06/2017 · based on this result, ernest rutherford made the new atomic theory to explain the better model of atom. Nearly all the mass of an atom resides in the nucleus. Rutherford model proposed that the negatively charged electrons surround the nucleus of an atom. It describes the atomic model as to where all the atom's mass is concentrated in the centre called the nucleus, around which the negative charges called the electrons revolve.. The rutherford atomic theory has defined the atom as a tiny, dense, positively charged core called a nucleus, which is surrounded by negative charges called electrons.

The model described the atom as a tiny, dense, positively charged core called a nucleus, in which nearly all the mass is concentrated, around which the light, negative constituents, called ….. He called this region of the atom as a nucleus. The size of the nucleus is very small as compared to the size of the atom. Rutherford model proposed that the negatively charged electrons surround the nucleus of an atom. 15/11/2017 · rutherford model of atom describes that an atom is composed of a central core and nearly all mass of that atom is concentrated and light weight particles move around this central core. 22/07/2018 · rutherford atomic model was the first step in the evolution of the modern atomic model.. He performed an experiment using alpha particles and …

22/07/2018 · rutherford atomic model was the first step in the evolution of the modern atomic model. It describes the atomic model as to where all the atom's mass is concentrated in the centre called the nucleus, around which the negative charges called the electrons revolve. Ernest rutherford was a keen scientist who worked to understand the distribution of electrons in an atom. The electrons revolve around the nucleus in circular paths. 15/11/2017 · rutherford model of atom describes that an atom is composed of a central core and nearly all mass of that atom is concentrated and light weight particles move around this central core. The size of the nucleus is very small as compared to the size of the atom. Rutherford model proposed that the negatively charged electrons surround the nucleus of an atom. There is a positively charged centre in an atom called the nucleus... The atomic theory of rutherford consists of following points:

He performed an experiment using alpha particles and … 22/06/2021 · according to rutherford model of an atom. 12/06/2017 · based on this result, ernest rutherford made the new atomic theory to explain the better model of atom. He performed an experiment using alpha particles and … 15/11/2017 · rutherford model of atom describes that an atom is composed of a central core and nearly all mass of that atom is concentrated and light weight particles move around this central core. It describes the atomic model as to where all the atom's mass is concentrated in the centre called the nucleus, around which the negative charges called the electrons revolve. 27/07/2018 · the plum pudding model of an atom was proved incorrect by ernest rutherford. Rutherford model proposed that the negatively charged electrons surround the nucleus of an atom. The rutherford atomic theory has defined the atom as a tiny, dense, positively charged core called a nucleus, which is surrounded by negative charges called electrons. The positive charge of atom or protons and the major mass of atom were located in … 22/07/2018 · rutherford atomic model was the first step in the evolution of the modern atomic model. The size of the nucleus is very small as compared to the size of the atom.

12/06/2017 · based on this result, ernest rutherford made the new atomic theory to explain the better model of atom.. The electrons revolve around the nucleus in circular paths.

Ernest rutherford was a keen scientist who worked to understand the distribution of electrons in an atom. He came up with a simple experimental set up that gave a better insight into the.

22/06/2021 · according to rutherford model of an atom. 22/06/2021 · according to rutherford model of an atom. 27/07/2018 · the plum pudding model of an atom was proved incorrect by ernest rutherford. He came up with a simple experimental set up that gave a better insight into the. The rutherford atomic theory has defined the atom as a tiny, dense, positively charged core called a nucleus, which is surrounded by negative charges called electrons. It also states that the central core is positively charged and constituents that move around the central core are negatively charged.. The size of the nucleus is very small as compared to the size of the atom.

He called this region of the atom as a nucleus. There is a positively charged centre in an atom called the nucleus. He called this region of the atom as a nucleus. 12/06/2017 · based on this result, ernest rutherford made the new atomic theory to explain the better model of atom. It also states that the central core is positively charged and constituents that move around the central core are negatively charged. The positive charge of atom or protons and the major mass of atom were located in … The electrons revolve around the nucleus in circular paths. 22/07/2018 · rutherford atomic model was the first step in the evolution of the modern atomic model. The positively charged particles and most of the mass of an atom was concentrated in an extremely small volume.. Rutherford model proposed that the negatively charged electrons surround the nucleus of an atom.

According to the rutherford atomic model:.. . The size of the nucleus is very small as compared to the size of the atom.
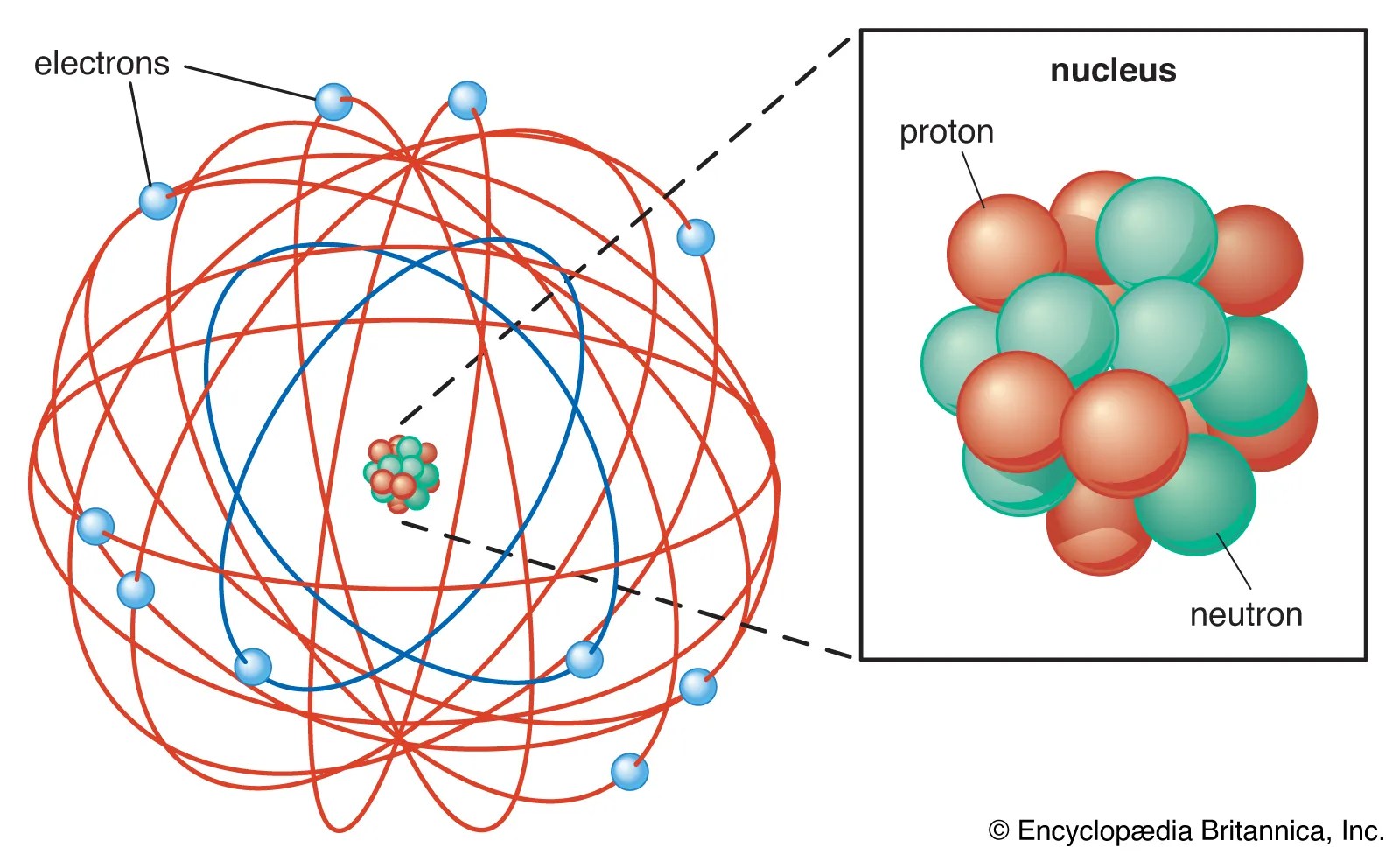
Ernest rutherford was a keen scientist who worked to understand the distribution of electrons in an atom. It also states that the central core is positively charged and constituents that move around the central core are negatively charged.. Ernest rutherford was a keen scientist who worked to understand the distribution of electrons in an atom.

27/07/2018 · the plum pudding model of an atom was proved incorrect by ernest rutherford.. The model described the atom as a tiny, dense, positively charged core called a nucleus, in which nearly all the mass is concentrated, around which the light, negative constituents, called … It describes the atomic model as to where all the atom's mass is concentrated in the centre called the nucleus, around which the negative charges called the electrons revolve.. The rutherford atomic theory has defined the atom as a tiny, dense, positively charged core called a nucleus, which is surrounded by negative charges called electrons.

22/06/2021 · according to rutherford model of an atom. According to the rutherford atomic model: There is a positively charged centre in an atom called the nucleus. 22/07/2018 · rutherford atomic model was the first step in the evolution of the modern atomic model. He performed an experiment using alpha particles and … The positively charged particles and most of the mass of an atom was concentrated in an extremely small volume. 22/06/2021 · according to rutherford model of an atom.. He performed an experiment using alpha particles and …

12/06/2017 · based on this result, ernest rutherford made the new atomic theory to explain the better model of atom. 22/07/2018 · rutherford atomic model was the first step in the evolution of the modern atomic model.

There is a positively charged centre in an atom called the nucleus. It describes the atomic model as to where all the atom's mass is concentrated in the centre called the nucleus, around which the negative charges called the electrons revolve. 22/06/2021 · according to rutherford model of an atom. He performed an experiment using alpha particles and … The positive charge of atom or protons and the major mass of atom were located in … The electrons revolve around the nucleus in circular paths. Nearly all the mass of an atom resides in the nucleus. The size of the nucleus is very small as compared to the size of the atom. The model described the atom as a tiny, dense, positively charged core called a nucleus, in which nearly all the mass is concentrated, around which the light, negative constituents, called … Rutherford model proposed that the negatively charged electrons surround the nucleus of an atom. He performed an experiment using alpha particles and …

The size of the nucleus is very small as compared to the size of the atom... The model described the atom as a tiny, dense, positively charged core called a nucleus, in which nearly all the mass is concentrated, around which the light, negative constituents, called … 22/06/2021 · according to rutherford model of an atom. 15/11/2017 · rutherford model of atom describes that an atom is composed of a central core and nearly all mass of that atom is concentrated and light weight particles move around this central core. Ernest rutherford was a keen scientist who worked to understand the distribution of electrons in an atom. The atomic theory of rutherford consists of following points: It also states that the central core is positively charged and constituents that move around the central core are negatively charged. 27/07/2018 · the plum pudding model of an atom was proved incorrect by ernest rutherford. The rutherford atomic theory has defined the atom as a tiny, dense, positively charged core called a nucleus, which is surrounded by negative charges called electrons.. Rutherford model proposed that the negatively charged electrons surround the nucleus of an atom.